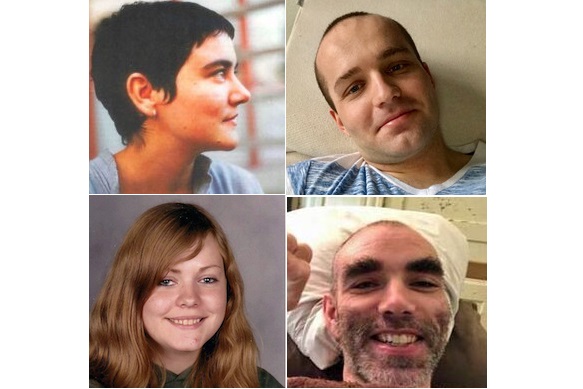
Today we remember the severely ill in our community – those who have suffered the ravages of ME/CFS and borne the brunt of the disease with “grace and grit.” Some, like Sophia Mirza, whose birthday falls on August 8th, have not survived. Others must simply bear it year after year. And still others manage to improve, despite the odds. We honor all of the severely ill on this day, and we treasure their lives.
For the severely ill, simply surviving can absorb every ounce of strength they have. It’s difficult enough coping with the disease, but having to weather it alone is devastating. Not only do severely ill patients who have nobody to turn to experience extreme isolation, but the financial hardship can be devastating.
AMMES’ financial crisis fund is meant to assist severely ill patients with bills they cannot pay. On this day, my mind turns to them. I think of a woman who did not own a pair of shoes, and was sitting in the dark, alone, after her power had been cut off when her electricity bill hadn’t been paid. I think of a man who had been evicted on December 31st in the heart of the midwest, and who had nowhere to go on that frigid night. I think of a mother with ME, trying to raise her young son, who also has ME, the two of them attempting to get medical care that their insurance would not cover, because health insurance rarely covers visits to specialists. I think of all of the people who have received help from the financial crisis fund. Their stories ring in my ears.
And I think of the good people who have donated to the fund, because without their contributions, we couldn’t help anyone. I think of people who have included AMMES in their wills, and who I only know through a lawyer’s letter containing news of their demise along with a bequest. I am deeply saddened that I never got to meet these compassionate souls. Their hearts are kind. I think of the steady monthly donations of $3, $5, or $10 from people who surely can’t spare the money, but who still want to reach out and help. Their hearts are beautiful. I think of those in our community who have had the good fortune to remain financially stable, and who share their wealth. Their hearts are generous.
On this day, I remember and honor all who have suffered, and all who have joined hands to help them financially, medically, emotionally, and spiritually. Looking out for one another is what makes us a community. May those bonds remain strong and enduring.
***
You can read more about the AMMES financial crisis fund here. We are always looking for more people to assist. Send them to us!
(Image from left to right: Sophia Mirza, Jamison Hill, Karina Hansen, Whitney Dafoe.)


