On this page you will find ongoing ME/CFS clinical trials looking for participants. For more ongoing clinical trials as well as completed clinical trials go to Clinicaltrials.gov
You can read about the latest resources for investigating the causes and mechanisms of ME/CFS here: New resources for large-scale ME/CFS research
Be sure to check the Institute for Neuro-Immune Medicine for their ongoing trials.
See the Icahn School of Medicine at Mt Sinai for their ongoing trials.
See Solve’s list of clinical trials for ongoing trials.
Stanford University is recruiting for a number of ongoing research studies.
Also see this google document for a long list of ongoing Long Covid trials: https://drive.google.com/file/d/1A_KYwsDR6_vzF8hqZTMhFan56Vzw-Gno/view
Hydrogen Water Intervention With Heart Rate Variability as an Outcome Biomarker in ME/CFS
The goal of this clinical trial is to learn if the OTC supplement, hydrogen water, works to treat the fatigue-related symptoms and functional limitations in adults with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). It will also examine if heart rate variability (HRV) can be used to predict who will benefit from the hydrogen water treatment. The main questions it aims to answer are:
Does the OTC supplement, hydrogen water, work to reduce the fatigue-related symptoms and improve functioning in participants who have ME/CFS?
Can HRV be used to predict who will benefit from treatment with hydrogen water?
MECFS research of potential treatment (Hydrogen Water): Volunteers needed. New study of OTC supplement for ME/CFS symptoms. Home-based study for people in U.S. & Canada. Participants paid up to $100. For more info contact fred.friedberg@stonybrookmedicine.edu
Low-Dose Naltrexone For ME/CFS: Dose-Finding
ClinicalTrials.gov ID NCT07285473
Sponsor University of Alabama at Birmingham
Information provided by Jarred Younger, University of Alabama at Birmingham (Responsible Party)
This exploratory clinical trial tests low-dose naltrexone (LDN) for the treatment of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). A remote trial approach is used, with eligibility open to the entire U.S.
Enhanced External Counterpulsation to Treat Long COVID-19 Fatigue (EXPECT)
ClinicalTrials.gov ID NCT05668039
Sponsor Sheba Medical Center
The goal of this double blind, outcome-assessor blind, randomized controlled trial, is to compare the effectiveness of external encounter counterpulsation (EECP) versus sham procedure in participants with long COVID-19 fatigue.
The main question[s] it aims to answer are:
Whether EECP improves fatigue score
Whether EECP improves quality of life, six-minute walk test, and endothelial function Participants will attend 15 sessions (1-hour each) of EECP during 5 weeks Researchers will compare EECP versus sham procedure for the above outcomes.
MGH Brian Fog Study Seeks ME/CFS Participants
MGH researchers are recruiting participants with diagnoses of ME/CFS for a study on brain fog. The study involves MRI, EEG, blood draw, and cognitive testing over two days (not necessarily consecutive) at the Martinos Center for Biological Imaging in Charlestown. Participants will receive $225 for completing the study, with a parking voucher provided or transportation fees covered up to $100. For more information, please email ebarringer@mgh.harvard.edu or call 617-726-8120.
NE3107 in Adults With Neurological Symptoms of Long COVID
ClinicalTrials.gov ID NCT06847191
Sponsor BioVie Inc.
Researchers will compare NE3107 to a placebo (a look-alike substance that contains no drug) to see if NE3107 works to treat neurocognitive and fatigue symptoms of long COVID.
Participants will:
Take NE3107 or a placebo twice daily for 84 days
Visit the clinic 5 times for checkups and tests and have a follow up phone call.
Study Contact
Name: Penelope Markham, PhD
Email: pmarkham@bioviepharma.com
Ampligen in Chronic Fatigue Syndrome
ClinicalTrials.gov ID NCT00215813
Sponsor AIM ImmunoTech Inc.
An Open-Label Study of Poly I: Poly C12U (Ampligen®) in Patients with Severely Debilitating Chronic Fatigue Syndrome (CFS)/Myalgic Encephalomyelitis (ME). The FDA approved the study for cost recovery. Patients enrolled in the study are responsible for costs related to the therapy, e.g., drug cost, infusion cost, cost of supplies, diagnostic and other laboratory testing.
2 locations: Nevada and North Carolina
Contact: Diane Young
Phone Number: 352-448-7797
Email: diane.young@aimimmuno.com
Harvard Collaboration: Seeking Participants for the Life Improvement Trial (LIFT)
The Mestinon/LDN LIFT study (Life Improvement Trial) is seeking eligible participants within 150 miles of Brigham and Women’s Hospital (BWH) at Harvard Medical School. Recruitment will be conducted through OMF’s StudyME registry and among patients within the Harvard Medical School system.
To be considered for this and other studies, please sign up for OMF’s StudyME today. If interested in this particular study, email jsquires1@bwh.harvard.edu in addition to signing up for StudyME.
Approximately 160 people will be included in this study. Participants may qualify to be in this study if:
You are male or female, between 18 and 65 years of age (inclusive).
Meet National Academy of Medicine Criteria (NAM), Canadian Consensus Criteria (CCC), and demonstrate orthostatic intolerance for diagnosis of ME/CFS.
Onset of symptoms before December 2019.
Can adhere to the study requirements for the length of the study.
Have a functioning smartphone.
It will take each participant approximately 3 months to complete this study, including 3 study visits at BWH and 4 virtual visits. Study tests include two exercise (SHAPE) tests, urine and blood collection, the use of a wearable device (Garmin Vivosmart 5), and bi-weekly questionnaires.
You will be randomly assigned to one of four groups for this study
Pyridostigmine(Mestinon) and Low Dose Naltrexone (LDN), or
Pyridostigmine and Placebo, or
Placebo and LDN, or
Placebo and PlaceboYou will take these drugs by mouth, with or without food, every day for the entire duration of the study. You will record the date and time(s) of dosing as well as any comments about how the dosing made you feel in your medication diary (will be provided).
For participating in this trial, you will receive a total of $300 USD by the final visit and will be allowed to keep the Garmin Vivosmart 5.
Center for Enervating NeuroImmune Disease
Location: Patient interactions will occur at the Hospital for Special Surgery (HSS, Manhattan, NY)
We are seeking volunteers comprising 40 ME/CFS patients and 20 controls to participate in this NIH funded (U54AI178855) project. The ME/CFS subjects will be identified by expert physician Dr. Susan Levine, M.D. (Manhattan, NY). Only those participants whose onset of ME/CFS was before 2020 will be eligible. Please review our recruitment flyer for more information on how to volunteer as a patient or control in this research study.
Location of Study:
Univ. Mass. Chan Medical School
Department of Medicine
Worcester, MA
Time Commitment: Adult Initial visit 30 min in person, two follow-up visits 30 min each in person; Children initial visit, 1 follow-up visit, optional joining study to follow up to 4 years.
Contact: Dr. Hayla Sluss
Phone: 508-856-3372
Email: Hayla.sluss@umassmed.edu
We are investigating the interplay of cellular landscapes and the role of oxidative stress and how they contribute to the disease in chronic conditions and illness.
We are seeking healthy volunteers or patients that self-report with these diseases:
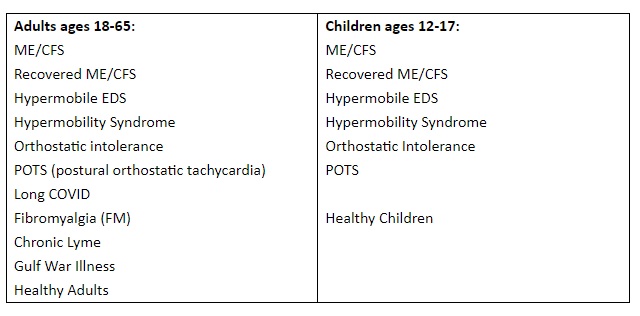


Read more HERE>> (Scroll down)
Open Medicine Foundation: Study ME
Our goal is to enhance the quality of life for individuals affected by ME/CFS, Long Covid, Fibromyalgia, and other post-infection illnesses by expediting research in these areas and increasing awareness by showing how many people are waiting for a cure. Signing up is easy and takes less than five minutes. All you need to do is provide your contact details and specify your areas of interest. Once you’ve done this, we’ll send you email notifications whenever there are research opportunities that align with your interests to potentially participate in surveys, laboratory studies, or treatment trials.
Pain and Fatigue Study Center at Mt. Sinai is conducting several studies
Patients with Long Covid and ME/CFS
The Pain and Fatigue Research Study Center at Mount Sinai is looking for patients suffering from Long COVID with severe fatigue to be in a 6-week trial of vagus nerve stimulation to potentially improve their health-related quality of life. The research study, to be done remotely, is designed to determine which of two possible investigational treatments produce the best outcome. There is no compensation. View study flyer here.
Eligibility Requirements
Female and Males between the ages of 21-70
Fulfill criteria for CFS or Long COVID
BMI of 30 and under
Further screening will determine your eligibility. If you are interested in possibly participating in this research study, please call us at 212-844-6665 or email us at PainandFatigue@mountsinai.org.
Patients with ME/CFS and Balance Problems
Many patients with ME/CFS have problems maintaining balance. This new study tests the individual patient for that problem and then applies a treatment — activation of the part that modulates balance via a battery-operated stimulator. The purpose of the study is to determine if this experimental treatment improves balance. If you are interested in participating in this study, please contact us either via email to info@painandfatigue.com or phone at 212-844-6665.
MRI Imaging to Compare Chronic Fatigue Syndrome Occurring With or Without Prior Covid-19 Infection
Many COVID-19 survivors remain ill with symptoms such as fatigue and brain fog. The symptoms are indicative of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). In this project, we will do magnetic resonance imaging of the brain to compare the images between long-COVID-ME/CFS and classic ME/CFS patients, as well as to individuals not affected by ME/CFS. Participants will be asked to undergo eligibility screening, complete a MRI scan, and answer questionnaires. Participants will be paid $100 upon completing the study. Knowledge learned from this study will deepen our understanding of ME/CFS/long-COVID disease mechanisms, aid in ME/CFS diagnosis, inform treatment decisions, and inspire new treatment targets. If you experience long-COVID or ME/CFS, or are a healthy individual, and are interested in participating in this study, please call 212-844-6665 or sign up at https://forms.gle/1uW36fMfjrFuNATX8
To examine why post activity fatigue, also known as post-exertional malaise (PEM) occurs in patients with myalgic encephalomyelitis/chronic fatigue syndrome [CFS] and at what level
Current thinking is two cardiac stress tests a day apart can be used to assess the common ME/CFS complaint of post-exertional malaise. Our government medical research agency, the NIH, has provided us funds to examine this link. One reason that exertion may trigger PEM might relate to reductions in blood volume which occur when a person has to rest. We are asking patients with ME/CFS as well as one healthy friend or relative to consider coming to Mount Sinai for testing. After we assure that you are either an ME/CFS patient or a healthy comparison person, we will determine your blood volume and then ask you to do two sequential cardiac stress tests. If your blood volume is reduced, you may get an infusion of saline designed to repair this deficit. Reimbursement for time and travel will be available. If you are interested in possibly participating in this study, please call us at 212-844-6665.
Researchers are studying possible treatments for adults who have Postural Orthostatic Tachycardia Syndrome (POTS) symptoms related to Long COVID. POTS causes a number of autonomic dysfunction symptoms like fast heart rate, dizziness, and fatigue when standing up from sitting or lying down to standing.
Sponsor: OMF
Study duration: 18 months
Conducted at The Harvard ME/CFS Collaboration at the Harvard Affiliated Hospitals
The study will examine EEG frequencies (electrical activity occurring in the brain) of sleep and wakefulness in ME/CFS patients. Concurrently, Dr. Jonas Bergquist will evaluate cerebrospinal fluid proteomics at the Uppsala Collaborative Research Center. Dr Bergquist hopes to identify orexin (a neuropeptide that regulates arousal, and wakefulness) and related proteomic inflammatory markers in patients who have developed ME/CFS.